健康・医療Information for Adverse Events
Information on Adverse Events associated with Beni-koji (Red Yeast Rice) related Products
If you have any of the following three products manufactured and sold by Kobayashi Pharmaceutical Co., Ltd. which are subject to a disposal order, please stop cosuming them immediately. If you have any symptoms or feel abnormalities, please consult with a medical doctor or contact a local public health center nearby your residence.
Products subject to disposal order:
1.Beni-koji Choleste-Help (45 tablets/15 days, 90 tablets/30 days, 60 tablets/20 days) 紅麹コレステヘルプ(45 粒 15 日分、90 粒 30 日分、60 粒 20 日分)
2.Naishi-Help Plus Cholesterol ナイシヘルプ+コレステロール
3.Nattou-Kinaze SaraSara tablet GOLD ナットウキナーゼさらさら粒 GOLD
Dietary Supplements containg Beni-koji produced and sold by Kobayashi Pharmaceutical Co., Ltd. (Violation for Article 6-2, Food Sanitation Act)

Pictures from Comsumer Affairs Agency Website
Summary
March 22, 2024
Kobayashi Pharmaceutical Co., Ltd. issued a press release titled "Request to stop the use of red yeast rice related products and notice of voluntary recall." This was in response to information reported to the company that kidney problems have occurred in those who consumed dietary supplements marketed by the company, and the result of analysis of the products and the raw materials conducted by the company showing, it was found that some red yeast rice may contain an unintended component.
March 26, 2024
In light of the report from Kobayashi Pharmaceutical Co., Ltd., the Ministry of Health, Labour and Welfare (MHLW) requested the local competent government to apply the violation of Article 6, Paragraph 2 of the Food Sanitation Act to these three products, and to order the company to dispose of these products according to Article 59 of the Food Sanitation Act. In response to the request, the local competent government ordered the company to recall these products.
April 5, 2024
In accordance with the opinion of the expert working group, the MHLW requested 52 companies to which Kobayashi Pharmaceutical Co., Ltd. sold Beni-koji raw materials and 173 companies that obtained Kobayashi Pharmaceutical Co., Ltd.'s Beni-koji raw materials through those 52 companies to conduct self-inspections on the followings:
(i) Are there any products that contain more than the same amount of Beni-koji as the three products in question (per day)? or
(ii) Even if condition (i) does not apply, are there any products for which one or more adverse events have been reported by medical doctors in the past three years?
As a result, none of the companies reported that they had any applicable products.
May 28, 2024
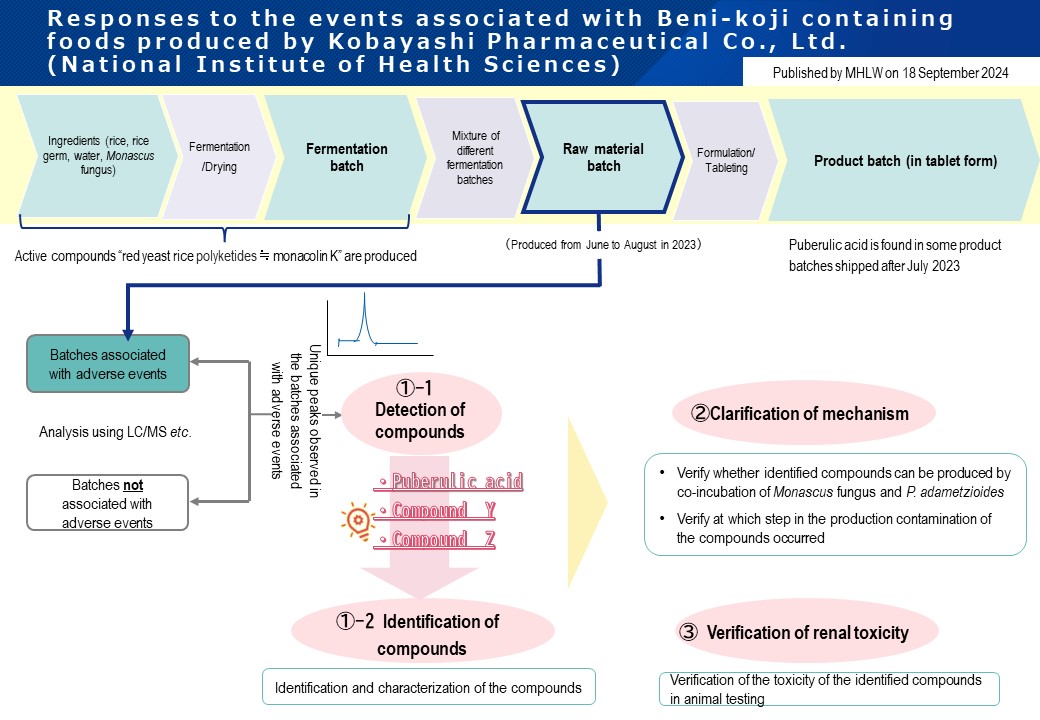
Investigations conducted by the MHLW in collaboration with the National Institute of Health Sciences have suggested the following:
- Puberulic acid, an unintended compound found in some Beni-koji raw materials used in the products that have been reported to be associated with adverse events, was produced by a certain blue mold (Penicillium adametzioides), and this blue mold was also involved in the production of two other compounds (Y and Z)
- Renal dysfunction was found in animal testing with puberulic acid alone and with the product (containing puberulic acid, compound Y and compound Z) associated with reported adverse events
The attribution of these compounds to renal dysfunction will be further investigated in animal testing.
September 18, 2024
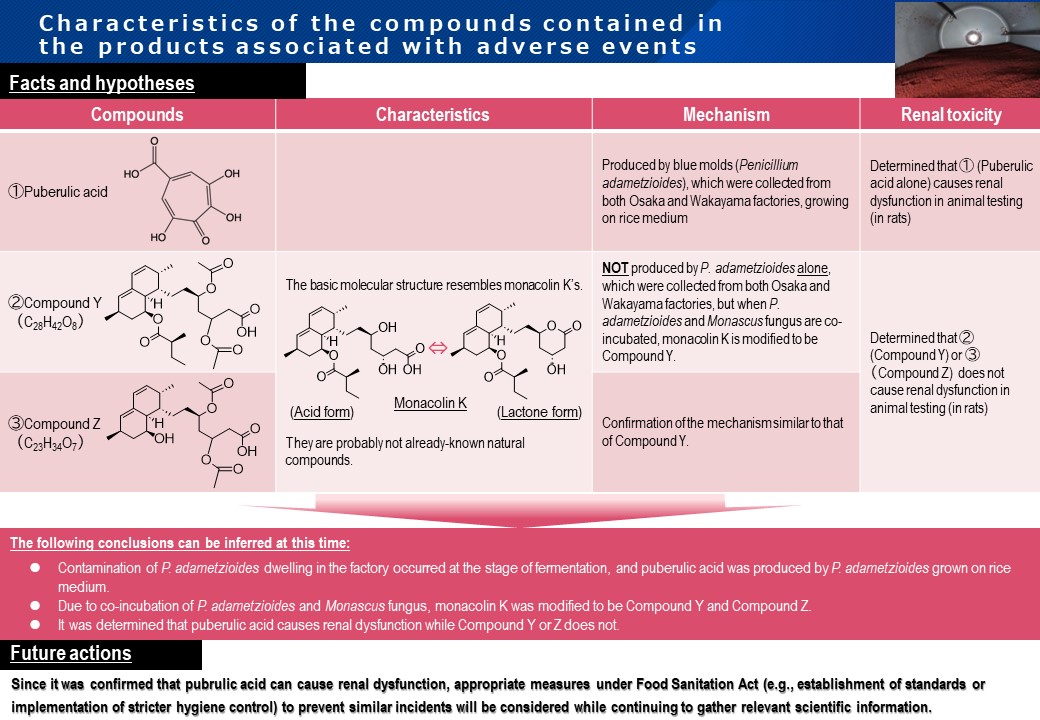
MHLW publicly explains the cause of the Beni-koji incident, which was investigated in collaboration with the National Institute of Health Sciences.
- Contamination of P. adametzioides dwelling in the factory occurred at the stage of fermentation, and puberulic acid was produced by P. adametzioides grown on ricemedium.
- Due to co-incubation of P. adametzioides and Monascus fungus, monacolin K was modified to be Compound Y and Compound Z.
- It was determined that puberulic acid can cause renal dysfunction, while Compound Y and Z do not.
Recall Information
Recall information on Foods containing Beni-koji manufactured by Kobayashi Pharmaceutical Co., Ltd.
Recall information submitted by food business operators to public health centers under the Voluntary Food Recall Reporting System is published on the MHLW's website, and can be searched using the "Public Recall Case Search''.
Of these, in addition to the three products subject to recall orders, MHLW has extracted products that are subject to voluntary recall as foods containing Beni-koji raw materials produced by Kobayashi Pharmaceutical Co., Ltd., as shown in the attachment.
For information on individual recalled products, please refer to the "Recall details" in the list.
List of Relevant Recall [22KB]
Reported Cases
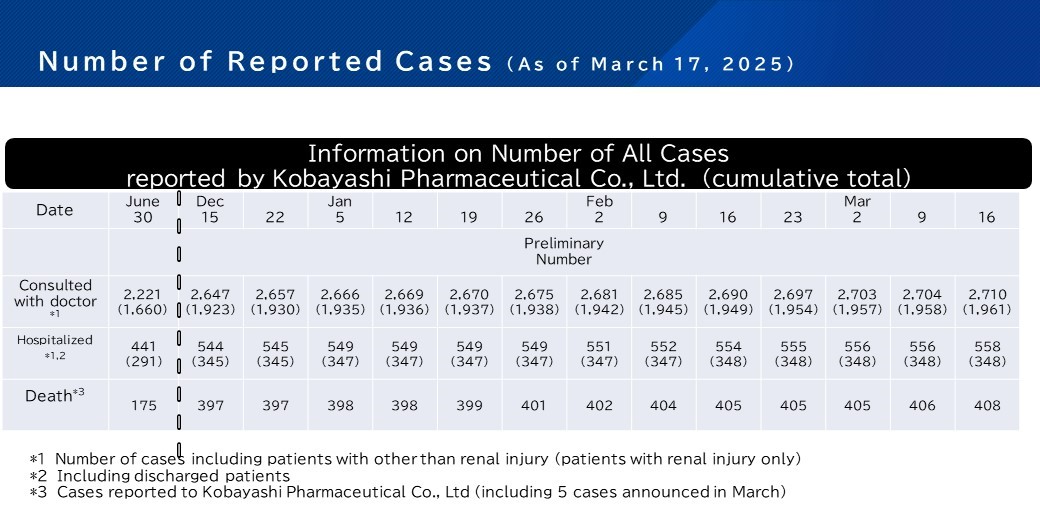
Osaka City published the final report on the cases regarding associated with Kobayashi Pharmaceutical Co., Ltd.’s Beni-koji related products on March 19, 2025.
MHLW will no longer update the number of cases after March 17, 2025.
The report is available at Osaka City website (Japanese only).
Current status of cause investigation
Company Announcement
(2nd Announcement) Request to stop the use of Beni-koji related products and notice of voluntary recall
(3rd Announcement) Request to stop the use of Beni-koji related products and notice of voluntary recall
(4th Announcement) Request to stop the use of Beni-koji related products and notice of voluntary recall
(5th Announcement) Request to stop the use of Beni-koji related products and notice of voluntary recall
(6th Announcement) Request to stop the use of Beni-koji related products and notice of voluntary recall
(7th Announcement) Request to stop the use of Beni-koji related products and notice of voluntary recall
(8th Announcement) Request to stop the use of Beni-koji related products and notice of voluntary recall
(9th Announcement) Request to stop the use of Beni-koji related products and notice of voluntary recall
(10th Announcement) Current adverse report of Beni-koji related products
Leaflet
Request for stop use of red yeast rice supplement by Kobayashi Pharmaceutical Co., Ltd.

Japanese[369KB]
English[291KB]
Chinese[284KB]
Korean[498KB]