Preventing the spread of infection and developing medical service systems
Gathering Medical Information System (G-MIS)
Gathering Medical Information System on COVID-19 (G-MIS)
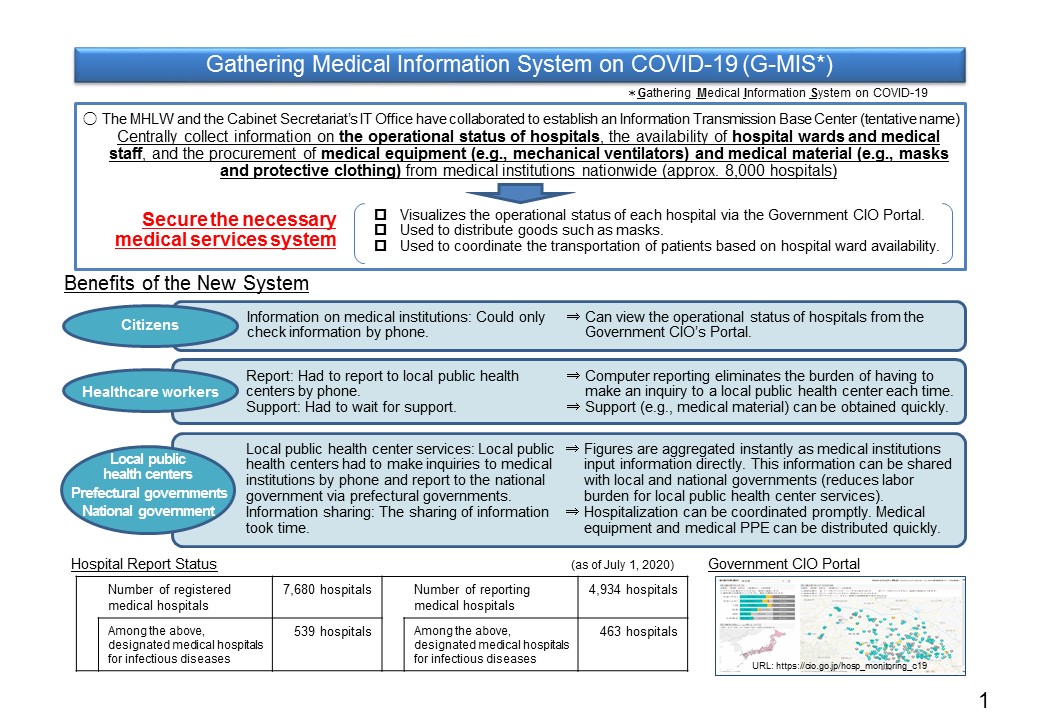
The MHLW and the Cabinet Secretariat's IT Office have collaborated to establish an Information Transmission Base Center (tentative name). The aim of this center is to make the operational status of hospitals widely known and help secure the necessary medical services system by, for instance, utilizing such information to distribute equipment (e.g., masks) and coordinate the transportation of patients. This is done by centrally collecting information on the operational status of hospitals, the availability of hospital wards and medical staff, and the procurement of medical equipment (e.g., mechanical ventilators) and medical personal protective equipment (e.g., masks and protective clothing) from medical institutions nationwide (approx. 8,000 hospitals with ≥ 20 beds).
This page provides information on the current status of outpatients, hospitalization, and emergency functions in hospitals.
Ascertaining the infection status (HER-SYS)
Health Center Real-time Information-sharing System on COVID-19 (HER-SYS)
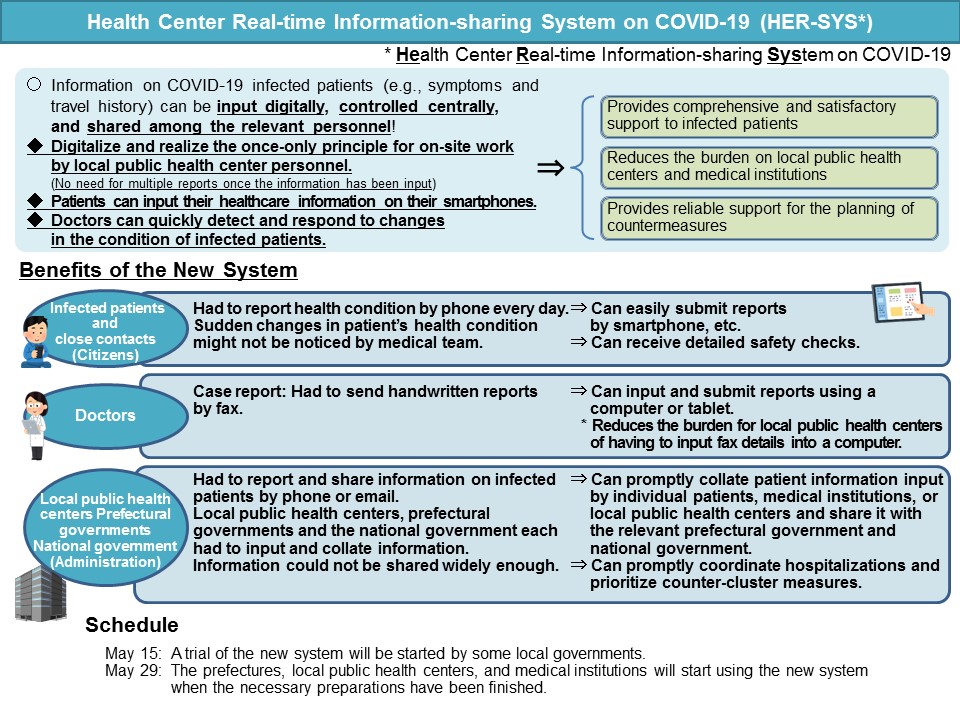
The MHLW has developed and implemented the Health Center Real-time Information-sharing System on COVID-19 (HER-SYS) to reduce the operational burden on local public health centers and accelerate information collection and sharing as an emergency plan.
This system allows information to be shared promptly among local public health centers, municipalities (departments other than public health centers), medical institutions, and other relevant parties (e.g., trustees for relevant operations).
※Health Center Real-time information-sharing System on COVID-19 (HER-SYS)
- To prevent outbreaks on COVID-19 (March 1)[PDF形式:150KB]
- Recommended Ventilation for Resolving “poor-ventilated enclosed spaces” (revised on April 3) [PDF形式:440KB]
- The MHLW publicly announced a recommended ventilation for resolving "poorly-ventilated enclosed spaces" with attention to heat illness prevention (June 24)[PDF形式:457KB]
Development of medications, vaccines, medical equipment, and test kits
Currently, a proven vaccine for COVID-19 has not yet been developed so symptomatic treatment is mainly used. Note that Remdesivir (Brand name: Veklury for intravenous injection 100 mg, etc.) got the Special Approval for Emergency * on May 7, 2020 (report on the Special Approval (Japanese only), package insert).
Therefore, the MHLW is utilizing various research funds, such as research funds from the Japan Agency for Medical Research and Development (AMED) and funds from the MHLW grants system, to develop medications for COVID-19. We are also continuing to expand studies on these agents. In addition to the study of vaccines in Japan, efforts are being made to secure the early development of a vaccine by promoting global cooperation through contributions to the Coalition for Epidemic Preparedness Innovation (CEPI).
In addition, several investigational agents for other treatments can be found among the existing medications, and observational studies, clinical studies, and trials are being promoted.
The MHLW is prioritizing and fast-tracking the examination of medical supplies, medical equipment (e.g., mechanical ventilators), and test kits for COVID-19. Click here for details.
Response to the Diamond Princess cruise ship
The infection control measures taken at the Cruise ship “Diamond Princess”(provisional translation)





