Introduction of the Positive List System for Agricultural Chemical Residues in Foods
Department of Food Safety, Ministry of Health, Labour and Welfare
June 2006
Department of Food Safety, Ministry of Health, Labour and Welfare
June 2006
I.Introduction of the positive list system
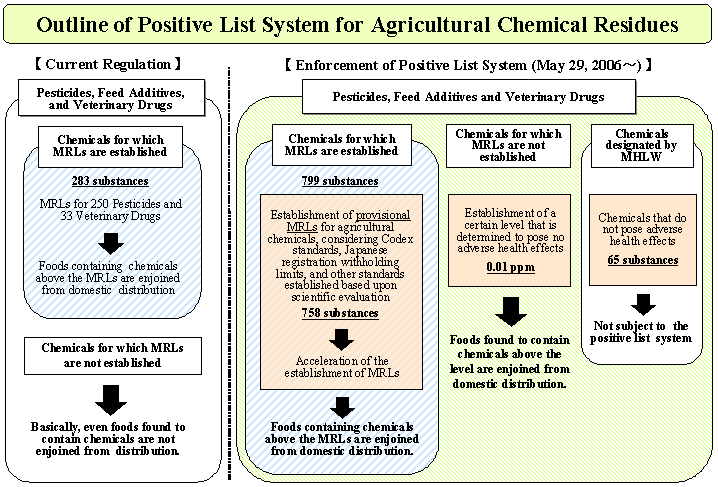
On May 29, 2006 the Ministry of Health, Labour and Welfare (MHLW) introduced the positive list system for agricultural chemicals remaining in foods-the system to prohibit the distribution of foods that contain agricultural chemicals above a certain level if maximum residue limits (MRLs) have not been established. The agricultural chemicals include pesticides, feed additives and veterinary drugs. This activity has been based on the Law to Partially Revise the Food Sanitation Law (Law No. 55, 2003). The Law No. 55 has required the MHLW to take the following measures within three years after the publication of the revised Food Sanitation Law (May 30, 2003).
Measures the Law required the MHLW to take
| i. | To establish a certain limit stipulated in Article 11 Paragraph 3 of the revised Food Sanitation Law* as a limit that is unlikely to pose adverse health effects (hereinafter referred to as the "uniform limit") |
| ii. | To designate substances stipulated in Article 11 Paragraph 3 of the revised FSL as those that will not pose adverse health effects (hereinafter referred to as "exempted substances") |
| iii. | To establish maximum residue limits stipulated in Article 11 Paragraph 1 of the FSL, which are provisionally established as compositional specifications for food (hereinafter referred to as "provisional MRLs")-as maximum levels of chemicals that can remain foods-in order to protect public health and smoothly implement the positive list system. |
The Pharmaceutical Affairs and Food Sanitation Council carried out discussions about provisional MRLs in and after June 2003. The MHLW published draft provisional MRLs (the first draft) in October 2003, and draft uniform level, exempted substances, and provisional MRL (the second draft) in August 2004, and their final draft in June 2005. It sought public comments about these drafts at home and abroad.
II.Discussion by the Food Safety Commission
Based on Article 23 Paragraph 1 (5) of the Food Safety Basic Law (Law No. 48, 2003), the Food Safety Commission (FSC) carried out discussions on the introduction of the positive list system for agricultural chemicals on the 14th and 21st of April 2005. On April 28, the Minister received the opinion from the FSC about some matters that should be kept in mind from the point of view of food safety.* These matters include the review of chemicals for which provisional MRLs are to be established and the formulation of a risk assessment program. On November 28, the Minister made a response to the chairman of the FSC concerning these matters.
III.Specifics of uniform limit, exempted substances, and provisional MRLs
A.Uniform limit
The uniform limit has been established for agricultural chemicals without MRLs. Basically, before a chemical is authorized, discussions are conducted on toxicity and other necessary matters. Based on the discussion results, restrictions are set on target crops and use amounts. Also, applications to the target crops and maximum limits that can remain in foods are established. This activity is conducted in the similar manner, regardless of country.
The MHLW has decided that it is appropriate to use a toxicological threshold of 1.5 µg/day as the basis to determine the uniform limit. The threshold has been based on the acceptable exposures, which have been used in evaluations of flavoring agents by JECFA (Joint FAO/WHO Expert Committee on Food Additives) and in evaluations of indirect additives by the US FDA (Food and Drug Administration) and on the ADIs (Acceptable Daily Intakes) of chemicals that had been already evaluated by JMPR (Joint FAO/WHO Expert Meeting on Pesticide Residues) or JECFA or in Japan.
The uniform limit has been set at 0.01 ppm so that the estimated intake of agricultural chemicals to which the limit would be applied does not exceed 1.5 µg/day when calculated based on the food consumption of Japanese population. In January 2005 the European Union (EU), which would introduce the positive list system, established the uniform level at 0.01 ppm. Considering such circumstances, the MHLW has decided that the limit is reasonable.
For chemicals for which the ADIs by JMPR or JECFA are extremely low, MRLs have been established at ND (not-detected level), instead of the uniform limits. For substances for which determination limits for analytical methods used in monitoring tests conducted by the Japanese local governments exceeds 0.01 ppm, the corresponding LOD of the analytical methods has been set.
B.Exempted substances
Exempted substances target pesticides, veterinary drugs, and feed additives which are used during the production of crops and aquatic products, and substances which are produced by chemical changes of these agricultural chemicals in food. The selection of exempted substances has been based on the following concepts when these agricultural chemicals themselves and their decomposition products could remain in food as a result of application in food production.
| i. | Agricultural chemicals and their decomposition products which are determined not to pose adverse health effects, given residue levels and forms, even if these chemicals remain in crops and animal products, including sea foods, to certain levels. |
| ii. | Specified agricultural chemicals shown in the Agricultural Chemicals Regulation Law, and chemicals for which registration withholding limits are not established and which are determined not to pose adverse health effects even if crops exposed to these chemicals are consumed. |
| iii. | Agricultural chemicals which are determined not to require any MRL in foreign countries and whose uses are not restricted. |
C.Provisional MRLs
MRLs which had been established before the FSL was revised in May 2003 did not cover all standards, including Codex standards and registration withholding limits for substances which are permitted for use in Japan. From the viewpoint of protection of public health and smooth implementation of the system, it was necessary to provisionally establish MRLs for chemicals without MRLs. In establishing provisional MRLs, Codex standards and other necessary information have been considered.
Provisional MRLs have been established taking into consideration:
| i. | Codex standards, |
| ii. | Registration withholding limits based on the Agricultural Chemicals Regulation Law, limits of determination for veterinary drugs at the time when they were authorized based on Pharmaceutical Affaires Law (Law No. 145, 1960), or limits of determination for feed additives at the time when they were authorized based on the Law for Safety Assurance and Quality Improvement of Animal Feed (Law No. 35, 1953), and |
| iii. | Standards established by countries where MRLs are assumed to be established based on toxicity study data equivalent in quality to those used in scientific evaluations by JMPR and JECFA. These countries are Australia, Canada, EU, New Zealand, and United States. |
For those chemicals categorized in either of the following two types, ND has been set instead of numerical limits: 1) genotoxic carcinogens and 2) chemicals that have been determined by JMPR or JECFA as those for which the ADI cannot be set. Separately from numerical limits, requirements/restrictions have been imposed on certain types of substances, including antibiotics, antibacterials, substances naturally occurring in foods, and chemicals for which standards are already set for food additive uses, and on applications of MRLs to processed foods.
The proposed provisional MRLs took effect on the date of implementation of the positive list system as compositional standards for food stipulated in Article 11 Paragraph 1 of the FSL. Basically, no changes has been made for MRLs already established based on Article 11 Paragraph 1.
<Progress of the activity>
October 2003
The First Draft published (draft provisional MRLs only)
August 2004
The Second Draft published (uniform level, exempted substances, and provisional MRL).
June 2005
The Final Draft published (uniform level, exempted substances, and provisional MRL).
October 24, 2005
As the result of discussion based on the above concepts, the Pharmaceutical Affairs and Food Sanitation Council reported to the Minister of Health, Labour and Welfare that
| (1) | the uniform limit should be established at 0.01 ppm, |
| (2) | 65 exempted substances should be specified, |
| (3) | provisional MRLs should be established for 758 chemicals. |
Notifications on the positive list system were published in the official gazette Kanpo:*
MHLW Notification No. 497 (Uniform Limit)(PDF:8KB)
MHLW Notification No. 498 (Exempted Substances)(PDF:10KB)
MHLW Notification No. 499 (Provisional MRLs, etc.)(PDF:479KB)(PDF:479KB)
May 29, 2006
The Positive List System took effect.
The MHLW intends, on a systematic basis, to ask the FSC to conduct safety evaluation for agricultural chemicals for which provisional MRLs have been established.
* Article 11 Paragraph 3 was newly added based on the Law to Partially Revise the Food Sanitation Law (Law No. 55, 2003). The added provision took effect on May 29, 2006.
* The opinion from the FSC (Japanese only) is available at:
http://www.fsc.go.jp/iinkai/positivelist_170428.pdf