The Call for Requests Related to Early Introduction of Highly Needed Medical Devices, etc. and Expanding the Scope of Applicability
Updated: May 23, 2016
Office of Medical Device Policy, Economic Affairs Division, Health Policy Bureau
Medical Device Evaluation Division, Pharmaceutical Safety and Environmental Health
Bureau
A call has been put out for requests for the development of medical devices and in-vitro diagnostics whose use has to date been approved in Western countries, etc. but not in Japan (hereafter, medical devices, etc.), and medical devices, etc. that have pharmaceutical approval, but some indications are unapproved in Japan although approved in Western countries. The “Study Group on Early Introduction of Highly Needed Medical Devices, etc.” (hereafter, Study Group) has assessed the medical necessity of submitted requests for development and promoted development by companies by selecting those with high medical need in Japan.
In addition, the Ministry of Health, Labour and Welfare (MHLW) drew up the “Strategy of SAKIGAKE” on June 17, 2014 to promote the practical implementation of innovative pharmaceutical products/medical devices, etc. As a part of this “Strategy of SAKIGAKE,” the “Scheme for Rapid Authorization of Unapproved Drugs,” which accelerates practical implementation within Japan, was applied to pharmaceutical products not yet approved in Western countries, etc. that satisfy certain conditions by expanding the examination focus of the “Study Group for Unapproved Drugs/Off-Label Drugs for High Medical Needs.” Similarly, the examination focus of the Study Group for Medical Devices, etc. was expanded to medical devices, etc. unapproved in Western countries, etc. that satisfy certain conditions, and the scheme to accelerate practical implementation in Japan was applied.
Consequently, as noted below, the scope of medical devices, etc. applicable to the call for requests for development has been expanded from existing medical devices, etc., and to accelerate examination by the Study Group, the call for requests for development and handling of requests by the Study Group has been reassessed based on past examinations, and a call for requests was put forth from October 1, 2015.
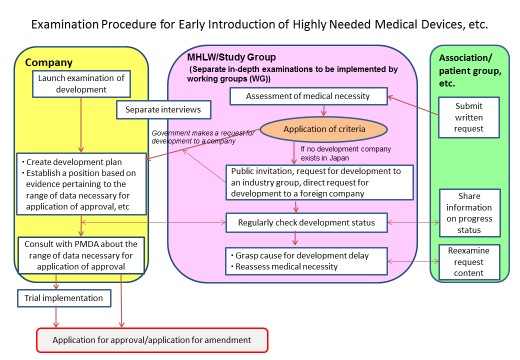
Refer to Figure 1 and Figure 2 for the scope of the newly added medical devices, etc. applicable to the call and the examination procedure of the Study Group.
Contact:
(Inquiries regarding submission of written requests, etc.)
Office of Medical Device Policy, Economic Affairs Division, Health Policy Bureau
Tel.: 03 (5253) 1111 ( Extension 2534)
(Other general inquiries regarding the Study Group)
Medical Device Evaluation Division, Pharmaceutical Safety and Environmental Health Bureau
Tel: 03 (5253) 1111 (Extension 4216)

1 Applicable medical devices, etc.
Requests for medical devices, etc. that fall under any of the following items (1)–(3) are being accepted.Note 1) Note 2)
(1) Medical devices, etc. approved in Western countries for which there is a high medical need Note 3)
(2) Off-label medical devices, etc. (medical devices, etc. that have pharmaceutical approval, but some indications are unapproved in Japan although approved in Western countries) for which there is a high medical need Note 3)
(3) Medical devices, etc. unapproved in Western countries that meet any of the requirements below for which there is a high medical need Note 3)
1. Medical devices, etc. that have had excellent test results published in a research paper, etc.
2. Medical devices, etc. for which an investigator-initiated clinical trial is being implemented or has been completed
3. Medical devices, etc. that have achieved steady results in an advanced medical care B program
Note 1) Separate from the existing applicable items (1) and (2), note that medical devices, etc. applicable to the newly added item 3) will be clearly distinct from the point of request submission due to the need to consider the procedure going forward.
Note 2) Medical devices, etc. applicable to the newly added item (3) will target medical devices, etc. developed either in Japan or abroad in order to accelerate practical implementation within Japan.
Note 3) Medical devices, etc. with a high medical need are those that correspond to criteria stated in 1. and 2. below.
1. Medical utility in any of the following instancesa. When treatment methods, preventive measures, or diagnostic methods do not exist
b. When medical utility as a treatment method, preventive measure, or diagnostic method can be anticipated from the perspective of efficacy, safety, physical/psychological patient burden, operability, etc. Note 4) Note 5)
2. Severity of applicable diseases in any of the following instancesa. A disease that has a serious impact on life (life-threatening disease)
b. When the progression of an illness is irreversible and the disease causes significant impact on day-to-day living
c. A disease that causes significant impact on others in day-to-day living
Note 4) This refers to requested medical devices, etc. corresponding to assessment criteria b for medical utility in cases where a decline in invasiveness, decrease in occurrence of complications, application to children due to miniaturization/weight reduction, improved safety/convenience of technique, improved endurance, improved operability, improved safety for medical workers, etc. can be anticipated compared to existing treatment methods, preventive measures or diagnostic methods.
Note 5) Regarding assessment criteria b for medical utility, in the case of applicable medical devices, etc. in (1) and (2), this refers to medical utility compared to existing treatment methods, preventive measures, or diagnostic methods indicated through clinical trial outcomes, etc. submitted at the time of approval in Western countries.
2 Application period
Applications will be accepted on a rolling basis from October 1, 2015.
3 Drawing up a request
(1) Use Appended Form 1, “Written Request for Early Introduction of Highly Needed Medical Devices, etc.” or Appended Form 2, “Summary Table for Development Request” to draw up a request.
Forms:
Appended Form 1: Written Request for Early Introduction of Highly Needed Medical Devices, etc. (MS-WORD) [437KB]
Appended Form 1 (Annex): Illustration of Overview (MS-PPT) [65KB]
Appended Form 2: Summary Table for Development Request (MS-EXCEL) [28KB]
※Because these forms are intended to be described by the Japanese academic society, no translated form is posted.
(2) Submit numbered copies of the literature and other reference materials as grounds for showing that request content is in the scope of applicable medical devices.
(3) Assessing the medical necessity of request content requires examining not only literature suggesting the validity of said request content, but also literature against the request content. Comprehensively investigate literature on the request content and incorporate it into the written request so that an appropriate assessment can be conducted.
(4) When there is a lack of literature, etc. indicating the applicability of the request content, note that to ensure smooth and swift clerical processing, in some cases resubmission is asked for prior to acceptance of submission or examination by a working group (hereafter, WG) or Study Group.
(5) In addition, in cases of resubmission of requests for which the Study Group could not necessarily conclude “high medical need” that did not go on to development/public announcement, provide specific data and materials forming the basis of argument that could change the assessment outcome, such as changes to medical necessity based on the previous assessment, and specify the changed area.
4 Submission method
*Submissions accepted from October 1, 2015*
Send submissions by email to the address below. If submission by email is difficult, submit by post.
When submitting a request by email, be sure to write in the subject line, “Request for Early Introduction of Unapproved Medical Device, etc.” When submitting by post, send via a method that proves delivery by registered post, etc., include electronic media (CD-R (RW) or DVD-R (RW) only), and write in red letters “Request for Early Introduction of Unapproved Medical Devices, etc. Enclosed” to the bottom left of the address. Send To:
・ By email
Email address kiki-needs@mhlw.go.jp
Office of Medical Device Policy, Economic Affairs Division, Health Policy Bureau, Ministry of Health, Labour and Welfare
・ By post
Office of Medical Device Policy, Economic Affairs Division, Health Policy Bureau, Ministry of Health, Labour and Welfare
1-2-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-8916
5 Handling requests in the Study Group
(1) Accepting requests
- Following submission of a request, the office will check the contents, and after holding an interview with the entity making the request, the request will be treated as having been accepted when it has been determined that the included materials, explanations, etc. are sufficient.
- When literature, etc. indicating the request content falls within the scope of applicable medical devices, etc. is insufficient, or when the explanation regarding the applicability to the call for medical devices, etc. is insufficient, reexamination of the request will be asked for prior to acceptance of the request.
- Submitted requests and details of the reexamination request will be publicized on the MHLW website, excluding the names of individuals, contact information, etc.
- When an application for approval has already been made for medical devices, etc. that have been requested in Japan, the said request will be treated as ineligible for examination.
- When a request is submitted by a patient group or individual, the views on the validity, etc. of said request will be heard from relevant associations noted in the written request and companies possessing development rights within Japan related to the request content when the existence of such companies can be confirmed. An examination will be conducted based on said request and acquired views. Therefore, when a request is submitted by a patient group or individual, ensure that views on medical need in clinical settings can be obtained.
- The Study Group will examine the medical need related to a request. However, detailed examination of evidence for individual requests will be conducted by WG established under the Study Group that are composed of experts, etc. in various fields of medical care.
- When a WG cannot come to a conclusion regarding the medical need related to a request even after a certain period of time has passed since acceptance of said request, the content of discussions occurring at that time in the WG will be reported to the Study Group.
- When the entity making the request is an association, from the perspective of fairness, for the time being persons of the Study Group who are administrators of said association (members of the board of directors or those with higher positions) will carry out an explanation of background, etc. related to said request, but will not participate in decisions regarding said request.
- Ordinarily, entities will not be individually contacted about the status of the examination of individual articles. However, in instances such as the need for additional concrete materials and data in the examination process, individual requests may be made.
- A WG may ask for explanations from a suitable individual from the requesting association, etc. as needed to confirm the present situation in greater detail (actual conditions of use in a clinical setting, development state, etc.) or to confirm the intent, etc. of the request content.
- Even if high medical need has been assessed, when evidence existing at the time of examination cannot provide sufficient confirmation of efficacy, etc. and additional evidence is necessary for approval of application, indicate the possibility of cooperation in gathering said evidence on Appended Form 1. If cooperation cannot be made in gathering additional evidence, note that said request may be ineligible for examination.
- Since requests are accepted for submission at all times, even requests that did not go on to development can be resubmitted when the changed areas are clearly stated and definitive changes to circumstances are recognized, such as newly added evidence.
- Development will proceed appropriately for medical devices, etc. that have been assessed as having a high medical need by the Study Group and for which a request for development has been made to companies or a public invitation has been issued to a development company, but instances of major changes to the medical environment from the time medical need was assessed are anticipated due to changes in standard treatment methods, etc.
- In such instances, application for reexamination will be accepted from companies, etc. carrying out development and medical need will be reexamined.
- When reexamination is conducted, presentation of materials indicating the decline in need for medical devices, etc. that are under development and changes to the medical environment related to medical necessity will be sought.
- The method of reexamination/publication of outcomes, etc. will be identical to the heretofore assessment of medical need. In addition, when medical need is determined not to be high at the time of reassessment, the request for development and public invitation to said companies will be withdrawn.
- When confirmation can be made of a company that possesses the development rights in Japan for the content of requests assessed to have high medical need by the Study Group, a request for development will be made to said company.
- When confirmation cannot be made of a company that possesses the development rights, a public invitation will be issued to development companies and a request for development made to related industry groups in Japan. In addition, a request for development will be made to overseas manufacturing companies for medical devices, etc. that are manufactured overseas.
*When examining request content, the Pharmaceuticals and Medical Devices Agency (PMDA) may contact relevant companies and associations, etc. that submitted the request.
To see the charts(pdf file), it requires a software "Acrobat Reader".
You can get Acrobat Reader for free, please click on the next button. Get Adobe Reader
