Standards and Evaluation Division
Department of Food safety
Ministry of Health, Labour and Welfare
the First Draft of Provisional Maximum Residue Limits for
Agricultural Chemicals in Foods
The Ministry of Health, Labour and Welfare (MHLW) invites your comments on draft provisional maximum residue limits (MRLs) for agricultural chemicals in foods.
The MHLW is going to implement a so-called "positive list system"- a system to prohibit the distribution of foods that contain agricultural chemicals above a certain level if maximum residue limits (MRLs) have not been established. The agricultural chemicals include pesticides, veterinary drugs and feed additives. This activity is based on the revised Food Sanitation Law published in May 2003. The system will take effect within three years after the publication of the revised law.
The first draft provisional MRLs are based upon the opinion from the Subcommittee on Pesticide and Veterinary Drugs of the Food Sanitation Committee under the Pharmaceutical Affairs and Food Sanitation Council, an advisory scientific expert group to the Minister. The list of draft provisional MRLs is attached to this document (this document can be found at: https://www.mhlw.go.jp/english/index.html)..
We are asking for your comments on the draft MRLs in order to facilitate the implementation of the positive list system. Please send your comments to us, according to the instruction given below. Please note that the MHLW will not respond individually to each comment.
Instruction for submission of commentsm
- Comment Period:From 28 October 2003 to 27 January 2004
Necessary information
>Please send your comments, accompanied by rationales including underlying documents, to the mailing address or e-mail address given below. Comments are preferably in Japanese.
>Make sure to include the specific title: "Comments on the first draft of provisional MRLs." Submissions by phone will not be acceptable.
>Please identify yourself by including your name, address, and occupation. In the case of a legal person (company or other organization), indicate the name and address of the legal person. Please note that submitted information, excluding your identity, may be made public.
Address
Mail: Standards and Evaluation Division
Department of Food Safety
Ministry of Health, Labour and Welfare
1-2-2 Kasumigaseki, Chiyoda-ku, Tokyo
100-8916, JAPANE-mail: positivelist@mhlw.go.jp
Outlines of subcommittee sessions on the implementation of the positive list system and accompanying documents are accessible at the MHLW web site. The English versions are not available.
- The Meeting of the Joint Subcommittee on Toxicology, Pesticide Residues and Animal Origin Foods of the Food Sanitation Committee, 27 June 2003
https://www.mhlw.go.jp/shingi/2003/06/s0627-22.html
Future business
The Subcommittee on Pesticides and Veterinary Drugs will conduct further discussion before the provisional MRLs are finalized, taking the obtained comments into consideration. The MHLW will then notify the WTO in accordance with the necessary formalities, and also seek public comments on the final draft provisional MRLs.
I. Background
The Ministry of Health, Labour and Welfare revised Food Sanitation Law in May 2003. Based on the revised Food Sanitation Law, the MHLW is going to implement the positive list system. The system is aimed at prohibiting the distribution of foods that contain agricultural chemicals above a certain level unless MRLs for the chemical on the food are established. This system will go into effect within three years after the publication of the revised Food Sanitation Law.
| Provisional Translation of the Article 11, Paragraph 3, of the revised Food Sanitation Law (newly established provision) Any food that contains, as a residue, an active ingredient of an agricultural chemical defined in the Agricultural Chemical Control Law, a feed additive defined in the Feed Additives Safety Control Law, or a veterinary drug defined in the Pharmaceutical Affairs Law (including a substance produced by a chemical change in the active ingredient and excluding any substance specified by the Minister of Health, Labour and Welfare as not posing any adverse health effects) at a level exceeding the amount that is established by the Minister of Health, Labour and Welfare as not posing any adverse health affects after hearing the opinion of the Pharmaceutical Affairs and Food Safety Council, shall not be produced, imported, processed, used, prepared, or stored for the purpose of sale, or sold. However, this provision shall not be applied in the case that the maximum residue limit is established for the chemical on the food, in accordance with the provision of Paragraph 1 in this article. |
After the positive list system goes into effect, a food that contains an agricultural chemical without MRLs will be prohibited from being distributed if the chemical residue is over a certain level. The introduction of this system in the current situation could result in unnecessary disruption in international food trade, because the number of chemicals with MRLs is small in Japan, compared with that of globally distributed chemicals. Therefore, it is necessary to provisionally establish MRLs before the implementation of the system. These provisional MRLs will be established for chemicals that are domestically authorized under the Agricultural Chemical Control Law, and chemicals for which Codex MRLs or other MRLs are established based on scientific evaluations.
The three related subcommittees under the Food Sanitation Committee have agreed that provisional MRLs should be established according to the following procedure, and that comments on the draft provisional MRLs be invited from various experts.
II.Establishment of the First Draft Provisional MRLs
1.Drafting Provisional MRLs
- (1)Procedures
> Take into account 1) the Codex MRL; 2) the registration withholding limits under the Agricultural Chemicals Law (for veterinary drugs, limits of quantification or determination (LOD) established at authorization under the Pharmaceutical Affairs Law; 3) the MRLs in foreign countries that have been based upon scientific toxicity evaluations required by the Joint FAO/WHO Meetings on Pesticide Residues (JMPR) and the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Australia, Canada, New Zealand, the USA, and European Union have kindly offered their cooperation in response to our request to the foreign delegations at the Food Safety Group meeting on 11 April 2003. > Follow the decision tree given in Figure 1. > Take necessary measures to prevent excessive regulation of substances that can remain in food as agricultural chemical residues, and that can also remain as contaminants derived from the natural environment. > Do not establish provisional MRLs for substances that are agricultural chemicals already regulated as food additives. > In addition, consider the following points.
Points for consideration in the establishment of provisional MRLs
| - | The current established MRLs will remain unchanged and continue to apply. |
| - | When an ADI cannot be established due to carcinogenecity or other reasons, the provisional MRLs should be established as "Not Detected (ND)," according to the manner that has so far been used. |
| - | The current standards for antibiotics and synthetic antibacterials should remain unchanged, but the scope of targeted foods should be harmonized with global standards. The Food Sanitation Law stipulates that foods shall not contain antibiotics and that meat, poultry, eggs, fish, and shellfish shall not contain synthetic antibacterials. |
| - | When chemicals have different active ingredients but remain in food in the form of the same metabolite or decomposed substance, provisional MRLs for these chemicals should be set for the metabolite or decomposed substance. Also, when chemicals have different active ingredients but substances that can be determined for these chemicals are all the same, provisional MRLs should be set for the substance targeted for determination. Example: MCPB and MCPB methyl (Herbicide) |
| - | According to the FAO guideline, one significant figure should be used. The figures should be rounded if necessary. |
| - | Adoption of foreign countries' MRLs |
(i)The mean value should be applied as the provisional MRL when MRLs from multiple countries are used for one crop
Example:| Food | PR- MRL | Ref. | MRL | WHL | Codex | US | AU | CA | EU | NZ |
| Orange | 3.3 -> 3 | FC | 1 | 6 | 3 | |||||
| Chinese cabbage | 0.036 -> 0.04 | FC | 0.02 | 0.04 | 0.05 |
(ii)When there is a big difference in MRLs used for one crop, the most appropriate value should be applied, taking the deviation into account. The simple mean value should not be used. However if there is a justifiable rational, it should be considered.
(iii)When only EU or one of these four countries sets a numerical value that is the LOD as the MRL, a certain uniform level that does not pose any adverse health effect should be applied in the positive list system.
| - | Individual limits should be harmonized among crops in the same food category, such as cereal grains and cruciferous vegetables. |
- (2)Legal Status of Provisional MRLs
The provisional MRLs will be applied as the legal standards based on Article 11 of the Food Sanitation Law, after the positive list system goes into effect (within three years after publication of the revised law, that is, not later than May 2006). The provisional MRLs will be published without distinctions between agricultural chemicals, veterinary drugs, and feed additives.
2.Reference MRLs
The following table shows information on MRLs that were referenced in the development of draft provisional MRLs.
| Source Country (Organization) |
Category | Number of Substances |
| Japan | Agricultural Chemicals with Registration Withholding Limits under the Agricultural Chemical Control Law | 231 as of June 2003 |
| Veterinary Drugs authorized under the Pharmaceutical Affairs Law (*) | 133 as of June 2003 | |
| Codex AlimentariousCommission | Agricultural Chemicals | 129 as of June 2003 |
| Veterinary Drugs | 49 as of June 2003 | |
| United States of America | Agricultural Chemicals | 326 as of April 2003 |
| Veterinary Drugs | 104 as of April 2002 | |
| Canada | Agricultural Chemicals | 151 as of June 2003 |
| Veterinary Drugs | 65 as of May 2003 | |
| European Union | Agricultural Chemicals | 158 as of February 2003 |
| Veterinary Drugs | 124 as of July 2003 | |
| Australia | Agricultural Chemicals and Veterinary Drugs (**) | 418 as March 2003 |
| New Zealand | Agricultural Chemicals and Veterinary Drugs (**) | 161 as of December 2003 |
| * | Limits of quantification or determination (LOD) provided by the MAFF are referred because veterinary drugs are authorized under the Pharmaceutical Affairs Law on the condition that no residue occurs in animals at the final stage. |
| ** | MRLs in Australia and New Zealand are not distinguished between agricultural chemicals and veterinary drugs. |
3.Food Categories for MRL setting
A food that has currently be categorized as "products other than above-mentioned products" will be separated from that category and newly categorized as a single food category when its consumption is considerably large (above one gram per person per day) and Codex standards are established for that food category. The new food categories will be implemented at the enforcement of the positive list system. The following are examples of separated categories:
| New Food Categories (daily consumption) | Current Category (daily consumption) |
| Qing-geng-cai (1.37 grams) ("pak-choi"-type Chinese cabbage) |
Other cruciferous vegetables (3.7 grams R 2.3 grams) |
| Nira (Chinese chive) (1.74 grams) | Other liliaceous vegetables (2.5 grams R 0.8 grams) |
| Bamboo shoots (2.05 grams) | Other vegetables (13.3 grams R 11.3 grams) |
4.Provisional Maximum Residue Limits (MRLs) on Processed Foods
Provisional MRLs for processed foods will be established only when Codex MRLs are established for them. Individual MRLs will not be established for other processed foods; processed foods can be distributed as long as they are made of ingredients that meet standards under the Food Sanitation Law.
5.Review of Provisional MRLs
The established provisional MRLs will be reviewed about every five years, according to changes in the reference MRLs used in setting provisional MRLs. In addition, the MRLs will be reviewed based upon the risk assessments obtained from toxicity studies. The reviews will be conducted in the order of priority based upon total diet studies.
6.Others
| (1) | Any future changes in reference MRLs used in setting provisional MRLs will be reflected in the final provisional MRLs if time permits in administrative formalities (i.e., if changes occur before WTO notification and the invitation of public comment). Changes include the addition and withdrawal of chemicals and target crops. |
| (2) | The MHLW will develop a system for establishing MRLs on certain crops for agricultural chemicals that are not used at all in Japan or are used only for limited crops, upon request from abroad. Around spring 2004, the MHLW will prepare a guideline describing procedures for application from abroad. |
| (3) | In light of comments obtained during the given period, formal MRLs (MRLs established pursuant to regular formalities) might be established before the enforcement of the positive list system. |
Figure 1.
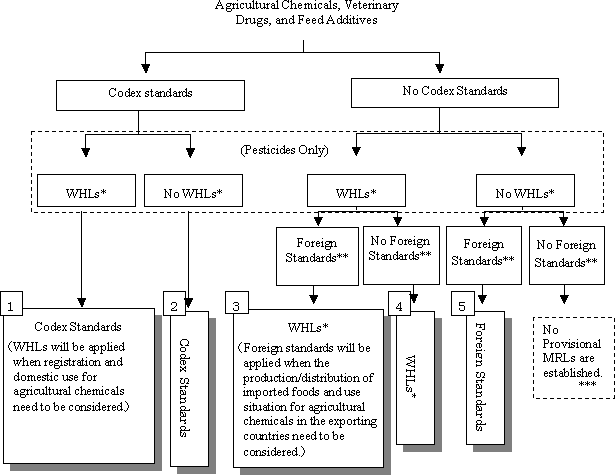
| * | WHLs: Registration Withholding Limits under the Agricultural Chemicals Control Laws. |
| ** | Foreign Standards: Standards in foreign countries that have been based upon scientific toxicity evaluations. |
| *** | When no provisional MRLs are established, a certain level of uniform limit that does not pose adverse health effects is applied. |
| : | Standards that will be applied as Provisional Standards |
Type
| 1. | Basically, the codex standard is applied, when both a Codex Standard and an WHL exist
|
||||||
| 2. | The Codex Standard is applied, when a Codex Standard exists but no WHL exists. | ||||||
| 3. | Basically, the WHL is applied, when both an WHL and one or more foreign standards exist.
|
||||||
| 4. | The WHL is applied when an WHL exists but no foreign standards exist. | ||||||
| 5. | The foreign standard (or the mean) is applied when no WHL exists but one or more foreign standards exist.
|
||||||
| 6. | Harmonization within the food category is done. |