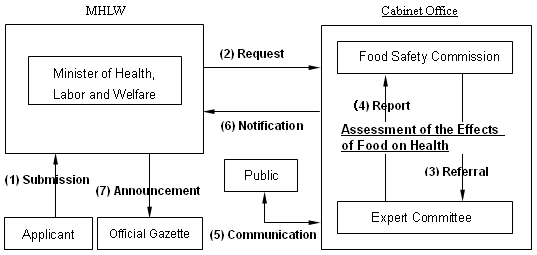
The safety assessment of foods and food additives produced by recombinant DNA techniques (hereafter GM foods) is mandatory under the Food Sanitation Law. The Ministry of Health, Labour, and Welfare (MHLW) receives application, and the Food Safety Commission (FSC) evaluates the safety in terms of human health.
FSC website: http://www.fsc.go.jp/english/index.html